Distal Mutations Rewire Allosteric Networks to Control Substrate Specificity in PTP1B #459
Authors
Xiaoyuan Wang, Ryan M. Anderson, Jinchan Liu, Victor Batista, J. Patrick Loria
Abstract
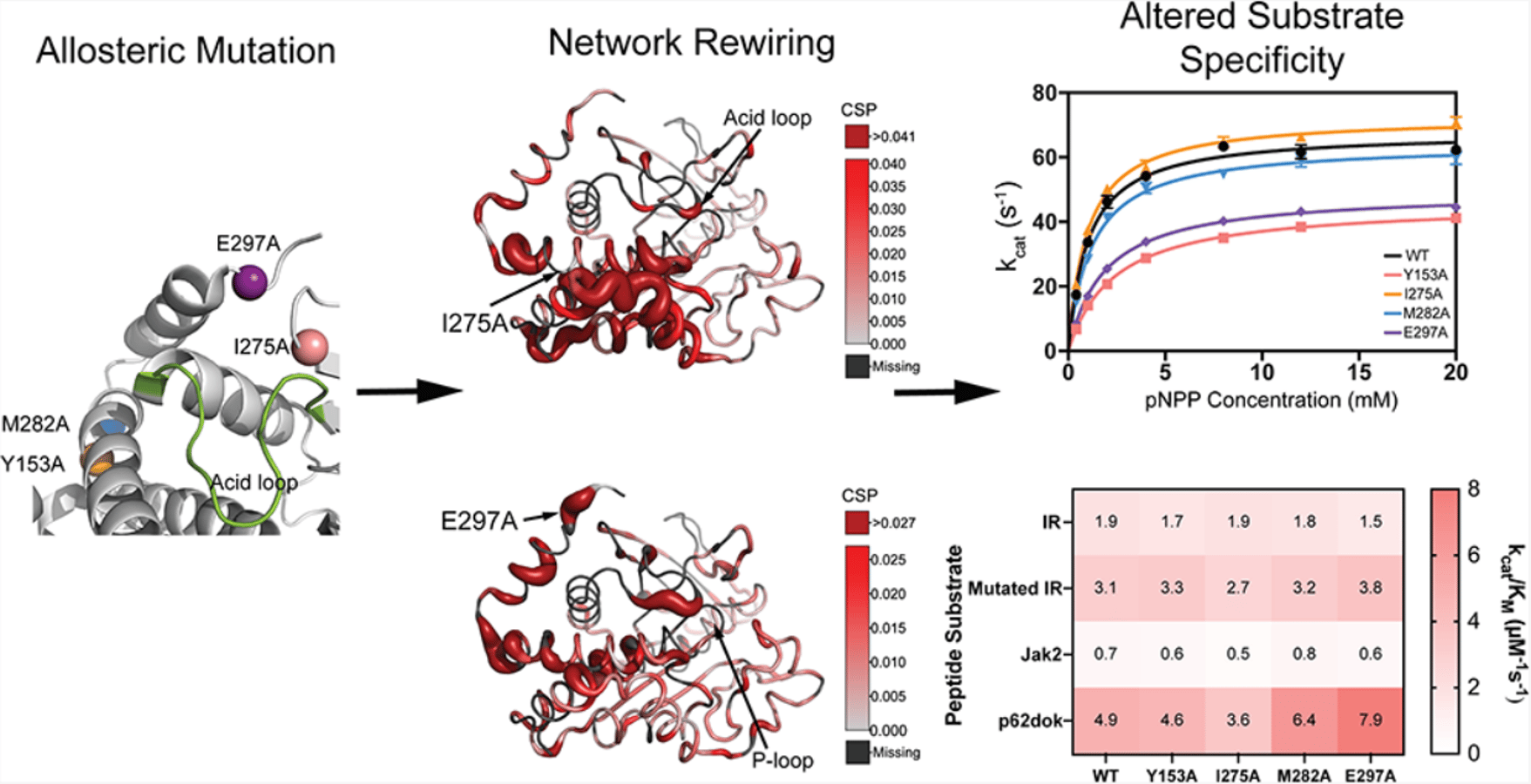
Protein tyrosine phosphatase 1B (PTP1B) is a key regulator of cellular signaling pathways, and its dysregulation is linked to diabetes, obesity, cancer, and immune dysfunction. While the catalytic mechanism of PTP1B is conserved across protein tyrosine phosphatases, its regulation by distal allosteric sites remains less understood. Here, we investigate how mutations at four allosteric sites (Y153, I275, M282, and E297) alter the PTP1B substrate specificity and enzymatic dynamics. Kinetic analyses with phosphotyrosine peptides and p-nitrophenylphosphate reveal that allosteric mutants display distinct changes in catalytic efficiency (k_{cat}/K_{m}), in some cases reversing substrate preference relative to the wild-type enzyme. Solution NMR spectroscopy and microsecond molecular dynamics simulations demonstrate that these mutations perturb long-range communication networks, disrupting coupling between helices α3 and α7 and altering acid-loop flexibility and active-site dynamics. Notably, the E297A mutation has the most pronounced effects, rigidifying the acid loop and weakening allosteric communication to the catalytic center. Community network analysis highlights the acid loop and helix α7 as central hubs linking distal sites to the active site. Together, these results establish that distal mutations can reshape PTP1B’s dynamic landscape, thereby modulating substrate specificity. This work expands our understanding of allosteric regulation in PTP1B and provides a framework for targeting dynamic networks to control phosphatase activity.