Electrochemical Deposition of an N-Heterocyclic Carbene (NHC) Functionalized CO2 Reduction Catalyst on Au Electrodes #456
Authors
Lydia R Weddle, Jan Paul Menzel, Pablo E Videla, Emile E DeLuca, Hao Fan Su, Hao Chen, Joseph M Palasz, James M Taylor, Zhuoran Long, Victor S Batista, Clifford P Kubiak
Abstract
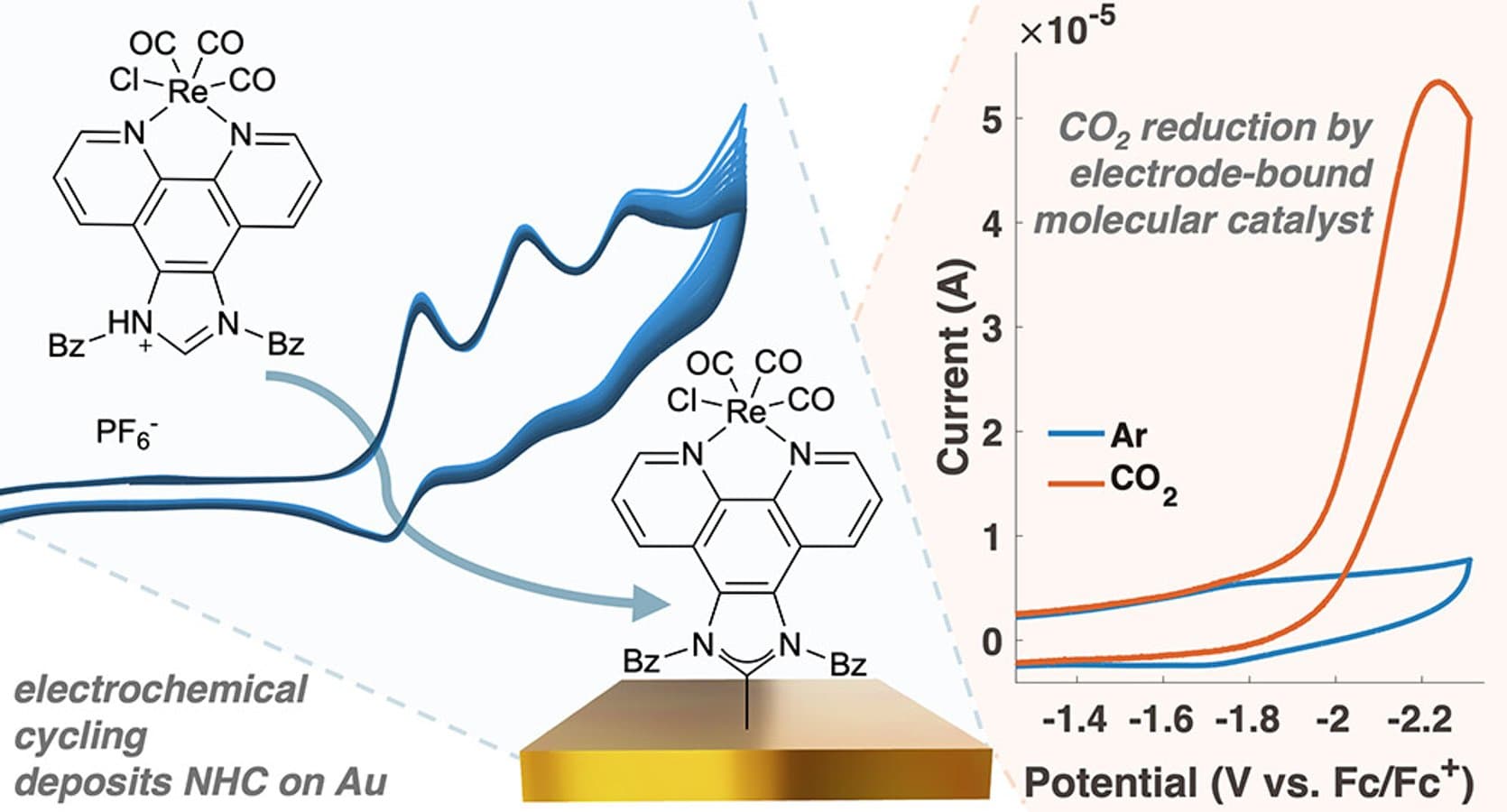
Electrode-bound molecular species provide a powerful platform for probing interfacial electric field effects on immobilized catalysts. However, common surface anchoring groups such as thiols and isocyanides largely undergo reductive stripping before catalytic onset. Herein, we demonstrate that stronger N-heterocyclic carbene (NHC) anchoring groups extend the accessible electrochemical window for studying catalysts immobilized on electrode surfaces. Specifically, we report the covalent attachment of an NHC-functionalized rhenium CO_2 reduction catalyst on gold via electrochemical deposition. Voltammetric characterization, polarization modulation infrared reflection absorption spectroscopy (PM-IRRAS), computational modeling, and X-ray photoelectron spectroscopy (XPS) confirm the formation of an electrochemically assembled monolayer that demonstrates persistence through large cathodic excursions and electrocatalytic CO_2 reduction.