Atomistic modulation of MIF‐2 structure, catalysis, and biological signaling via cysteine residues and a small molecule, Ebselen #455
Authors
Vinnie Widjaja, Sirena M D'Orazio, Pragnya Das, Divya T Rajendran, Xander Takada, Yuanjun Shi, Iz Varghese, Yannie Lam, Nicholas DaSilva, Jimin Wang, Victor S Batista, Vineet Bhandari, George P Lisi
Abstract
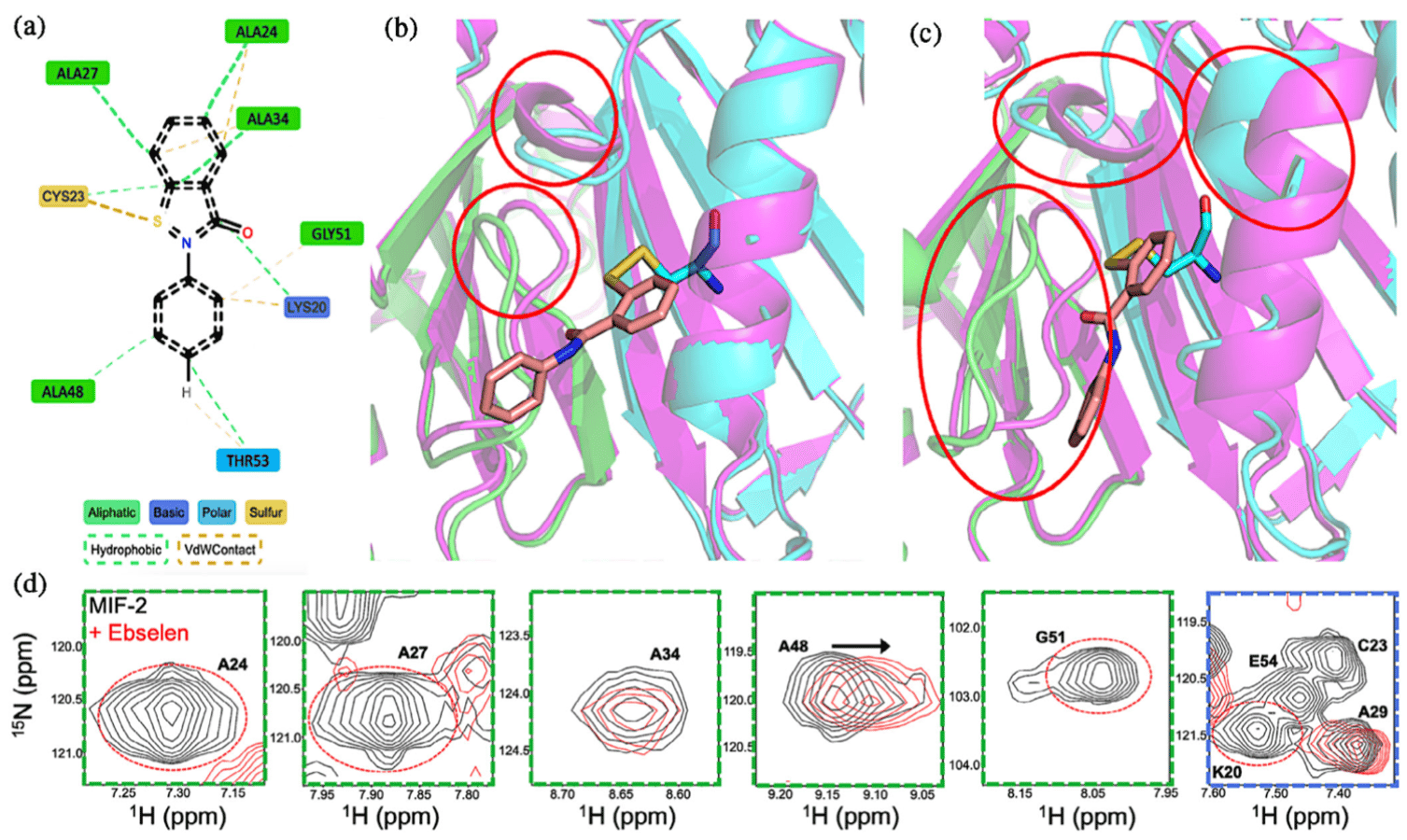
The macrophage migration inhibitory factor (MIF) family of cytokines comprised of the MIF and D-dopachrome tautomerase (or MIF-2) paralogs share identical tertiary and quaternary structures that contribute to their overlapping enzymatic and signaling activities. Recent investigations of MIF and MIF-2 have shown them to possess N-to-C-terminal allosteric crosstalk, but despite the similarity of this “allosteric pathway,” its regulation of MIF and MIF-2 is not identical. Thus, structure alone does not preserve the precise allosteric mechanism, and additional residues that modulate MIF and MIF-2 function must be characterized. Cysteines have been identified as allosteric switches for the same biochemical functions of MIF, and small molecules targeting its N-terminal enzymatic site have affected the structure of three proximal cysteines. Ebselen is a compound that forms covalent selenylsulfide bonds with MIF cysteines and is hypothesized to destabilize and dissociate the MIF trimer into monomers. Ebselen-bound MIF also displays little-to-no catalysis or biological signaling. However, it is unclear whether Ebselen similarly affects the MIF-2 paralog, despite MIF-2 containing two related cysteines (MIF contains three). We used mutagenesis, nuclear magnetic resonance, molecular dynamics simulations, in vitro and in vivo biochemistry to investigate the mechanism of Ebselen as a modulator of MIF-2 via its cysteines. Our findings suggest that Ebselen partially disrupts the MIF-2 homotrimer, though the overall population of such a structure is <35%, even on the timescale of many hours. Ebselen does attenuate the biological functions of MIF-2, and solution structural biology captures the conformational transitions preceding the destabilized MIF-2 trimer.