Nitrate reduction to ammonia catalyzed by GaN/Si photoelectrodes with metal clusters #439
Authors
Wan Jae Dong, Jan Paul Menzel, Kejian Li, Zhengwei Ye, Zhuoran Long, Ishtiaque Ahmed Navid, Ke R. Yang, Yixin Xiao, Victor S. Batista, Zetian Mi
Abstract
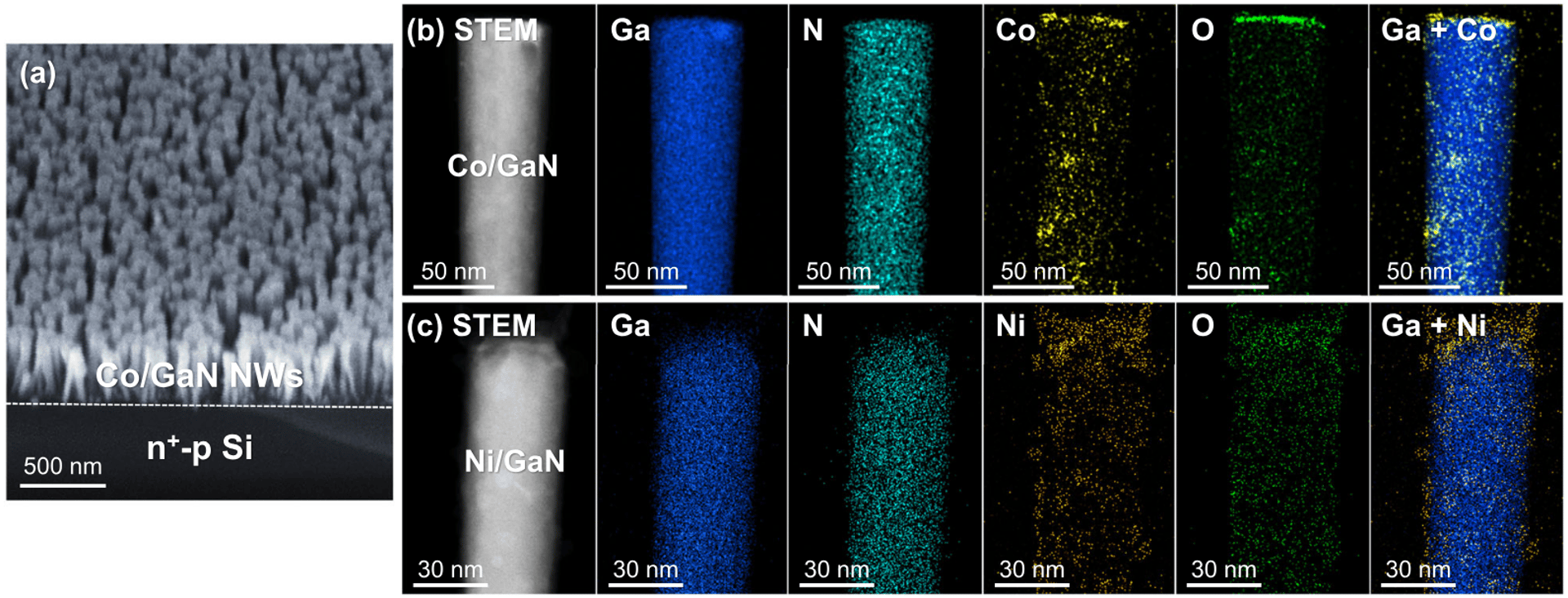
The development of photoelectrochemical cells for reduction of nitrate to ammonia under solar light is of significant interest for the production of clean chemicals and fuels but has remained a daunting challenge. Here, we investigate various metal catalysts supported on GaN nanowires grown on n^+ -p Si wafer – an emerging functional platform for scalable artificial photosynthesis – and demonstrate highly stable and efficient photoelectrochemical nitrate reduction reaction. We and that Co and Ni catalysts on GaN/Si exhibit the best performance, with an onset potential >0.3 VRHE and a faradaic efficiency of NH3 of 99% at 0.2 VRHE. These results highlight the advantage of photoelectrochemical system in achieving efficient nitrate reduction under more positive potentials. In-situ measurements and theoretical calculations reveal that the binding modes of the NO2 intermediate play a key role in the NH3 synthetic process. These results demonstrate that the rational design of catalysts on photoelectrodes can construct synergistic metal-semiconductor interactions for efficient and stable photoelectrochemical NH3 synthesis.